
New hippocampal circuitry of object-location memory identified…Functional connectivity patterns of thalamic nuclei mapped to better understand epilepsy…Two-photon and mesoscale imaging combined to examine local and global cortical networks…
Modern viral genetic-based brain mapping and imaging tools uncover a new cortico-hippocampal pathway
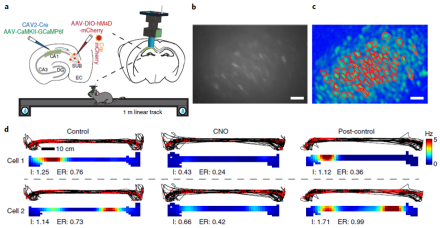
Historically, research on the hippocampal circuitry of spatial cognition and episodic memory has focused on the trisynaptic pathway – a relay loop in the hippocampus that shares connections with other brain areas, including the cortex. Recent studies have identified a back-projection within this loop from the subiculum (SUB) to the CA1. However, elucidating the precise organization and function of this pathway has been limited by a lack of effective circuit mapping tools. With funding from a BRAIN Initiative grant, a research team led by Dr. Xiangmin Xu recently overcame this technological challenge by developing a novel viral vector to anterogradely label neurons trans-synaptically. Here, Dr. Xu and his team at the University of California, Irvine (UCI) School of Medicine used this herpes-based virus and other modern genetic-based neurotechnologies to identify a new cortical-hippocampal circuit in mice. First, the viral tracer was paired with a monosynaptic rabies retrograde tracer to uncover a previously unknown connection between visual cortex and CA1 (via the SUB-CA1 pathway). Then, Xu and his colleagues used DREADDs (designer receptors exclusively activated by designer drugs) and optogenetics to inhibit or excite the pathway during behavior, revealing a functional role of SUB-CA1 neurons in object-location learning and memory. Researchers then wondered how exactly the SUB-CA1 pathway controlled this cognitive process at the circuit level. To address this, they combined in vivo calcium imaging via miniaturized fluorescence microscopes in CA1 with inhibitory DREADDs expressed in SUB-CA1 neurons to show that this pathway modulates the location-specific activity of neurons in its target region (the CA1). This work demonstrates how integrating newly developed brain mapping tools with emerging neuroscience technologies can advance our knowledge of both the anatomy and function of canonical neural circuits. The loss of object-location memory is a hallmark impairment in Alzheimer’s disease. These results may provide a new neural target to prevent the development of and lead to potential treatments for Alzheimer’s-related memory impairments. For more information, please read the UCI News Release and AAAS EurekAlert! News Release on Xu’s findings.
***
Connectivity patterns of thalamic nuclei predict seizure frequency in epileptic patients
Medial temporal lobe epilepsy (mTLE) is often treated pharmacologically. But anti-epilepsy drugs are not always ideal because some patients experience uncontrollable seizures and/or adverse drug-related side effects. For such cases, thalamic neurostimulation therapy is a popular treatment. However, therapeutic advancements in thalamic neurostimulation have been hindered by a limited understanding of the thalamic connectivity. The thalamus is a tiny structure composed of multiple nuclei with diverse functions and connections to and from other brain areas, creating complexities in mapping its circuitry and connections. To better understand the thalamic network, Dr. Gregory Worrell and his research team at the Mayo Clinic used advanced nucleus-specific functional magnetic resonance imaging (fMRI) methods to create a detailed map of the connectivity between the cortex and distinct thalamic nuclei. Researchers acquired high-resolution structural whole-brain images and resting-state fMRI data from 17 patients with mTLE and 17 healthy control participants. The fMRI data was then used to compute resting state functional connectivity (RSFC) between thalamic nuclei and various brain regions. When comparing mTLE patients and normal subjects, these brain maps revealed numerous differences in functional connections between individual thalamic nuclei and cingulate, frontal, parietal, and sensory-motor areas. Next, by analyzing a subset of RSFC data, Dr. Worrell and his team found that connectivity of some thalamic nuclei and seizure frequency were correlated in patients with drug-resistant mTLE. Results suggested that specific mediodorsal thalamic nuclei – common targets for neurostimulation therapy – may indirectly regulate seizures through connections with the limbic system. Researchers also found that the connectivity of motor- and sensory-relay nuclei may help elucidate sensory-motor deficits seen in patients with chronic seizures. This work provides fundamental insight into our understanding of basic neurophysiology and may inform novel treatment approaches for epilepsy and other disorders.
Scientists simultaneously image the cortex across microcircuit and global network scales
In the cortex, single neurons integrate signals from both local circuits and long-range projections originating in distant brain regions. Although anatomical, electrophysiological, and imaging studies have elucidated important facets of cortical networks across scales, directly relating local circuit function to global brain activity has been technologically elusive. Here, Dr. Michael Crair and Dr. Michael Higley, along with an interdisciplinary multi-lab team of neuroscientists at Yale University, developed a method to simultaneously measure the local microcircuit activity of single neurons and the meso-scale activity in the mouse cortex. To achieve this, researchers unified two technologies: two-photon microscopy to capture the activity of single neurons and mesoscopic imaging to measure widespread patterns of cortical network activity. The method involves a microscope with a ‘dual-axis’ design where a mesoscope objective is positioned above the brain and a two-photon objective with an ultra-long working distance (20 mm) is placed tangential to the skull. Imaging is performed through a specialized microprism that allows imaging with either system. Crair and his team used their new method to examine the somatosensory cortex (S1) and, based on local circuit activity, they found that individual S1 neurons differed in their large-scale connections. Then, researchers leveraged the method to study vasoactive intestinal polypeptide-expressing interneurons (VIP-INs) – a sparse (and notoriously difficult to image) neuronal population. They identified a new ‘arousal network’ by linking the activity of VIP-INs and pyramidal neurons, as well as their broad connectivity patterns, to mouse whisking behavior. To optimize their method, they also developed a viral transduction approach for robust whole-brain expression of genetically encoded calcium indicators. This powerful, multi-scale imaging tool will greatly accelerate our ability to measure and understand brain architecture and function across spatiotemporal scales. To read more about this collaborative method developed by Yale scientists, please read the Yale News Release.
