Creating a 3D atlas of nerve innervation in the pancreas…Instinct and experience drive the cortical plasticity needed for maternal behavior…A new central amygdala fear circuit…Shedding light on visual discrimination circuits in primates….
Scientists create a whole-organ 3D atlas of pancreatic nerve fibers in diabetes
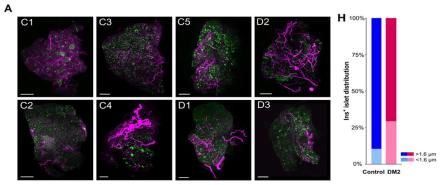
Islets are groups of cells in the pancreas that are important for glucose metabolism. Pancreatic islets contain several types of cells, including insulin-producing β cells. Previous studies have shown that islet cells receive dense input from nerve fibers and that this innervation controls blood glucose; thus, elucidating the detailed anatomy of the pancreas in diabetes may provide new insights into metabolic disease. The pancreas, however, is highly heterogeneous and our understanding of its anatomy and function remains incomplete. Here, Dr. Sarah Stanley and her research team at the Icahn School of Medicine at Mount Sinai used tissue clearing and whole-organ imaging to create a high-resolution 3D atlas of pancreatic nerve fibers and identified a new mechanism of neural input remodeling in diabetes. Specifically, researchers used the tissue clearing technique iDISCO+ and 3D volume imaging and analysis to determine the distribution of β cells, glucagon-producing α cells, and neurofilament 200 kDa (NF200)-positive input fibers in the pancreata of healthy and diabetic mice. NF200 is a neuronal marker that is thought to reflect neural remodeling. They also examined islet and nerve structure in pancreatic tissue from five healthy and three diabetic human donors. Dr. Stanley and her colleagues found that the innervation of the endocrine pancreas was enriched in healthy mice, and innervated islets were larger than non-innervated islets in healthy mice and humans. Islet nerve density and NF200 were increased in the islets of two mouse models of diabetes. Also, nerve density and the proportion of innervated islets was increased in pancreatic tissue from diabetic humans compared to healthy subjects. Researchers also suspected that nerve innervation may change during the development of diabetes. To address this, they used streptozotocin (STZ), a drug that is toxic to β cells, in mice to induce diabetes and found that islet nerve density increased over time compared to control mice. Overall, these findings show that nerve input into islets is maintained in diabetic humans and may be remodeled during disease development. This work constitutes a “3D atlas of pancreatic nerve innervation”, serving as a tool for researchers to quantify β cell mass, define islets, map pancreatic innervation, and assess the interaction between islets and innervation across species. Altogether, this work may help further our understanding of diabetes and lead to novel treatment targets.
Maternal responses to the cries of pups are driven by both innate and plastic mechanisms
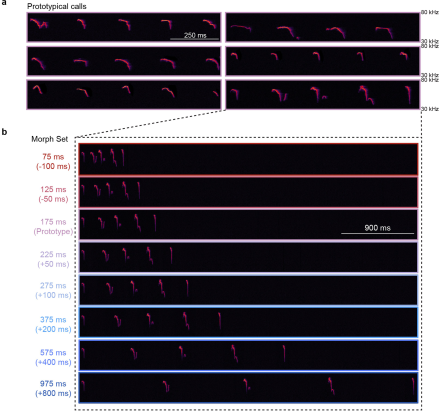
The brains of mammals seem to be hardwired to respond to the cries of our infants. However, a virgin mouse will not respond to the cries of another’s pups until she is co-housed with a dam and her litter. This suggests that there may be a re-wiring of the auditory cortex during this experience that enables pup retrieval behavior. But what enables this tuning of the brain to the cries of pups? Here, the lab of Dr. Robert Froemke and his team at New York University recently demonstrated that after co-housing with pups, cortical plasticity and the oxytocin system rapidly re-tuned neurons in the auditory cortex of virgin mice. The virgin mice also learned to recognize pup cries and retrieve pups. Thus, neural mechanisms triggered by pup experience build upon hard-wired instincts, and drive parenting behavior. In the study, researchers co-housed virgin female mice with a dam and her litter, and then assessed cortical re-tuning. First, they determined the inter-syllable intervals at which females responded to pups and established a library of both prototypical pup calls and morphed calls for later playback procedures. Then, using two-photon imaging and in vivo voltage-clamp recordings, they found that excitatory and inhibitory tuning and synaptic responses were altered by maternal experience. Next, they expressed the calcium indicator GCaMP6f in either excitatory or inhibitory neurons in the auditory cortex of virgins and monitored temporal tuning throughout co-housing. This revealed that co-housing results in the coordinated plasticity of neuronal tuning. Finally, the researchers optogenetically inhibited hypothalamic oxytocin neurons of virgin females, co-housed those females with a dam and her litter, during the playback of various pup calls. They found that the oxytocin system is required for the re-tuning of cortical neurons. Altogether, these data demonstrate the importance of central oxytocin in synaptic plasticity processes within the auditory cortex for maternal behavior. Further, this study illustrates how the cortex tunes to the vocalizations of pups. While both experience and innate mechanisms are necessary for the development of maternal behavior, these data provide new insight into how the brain learns maternal skills. To learn more about this study, check out the AAAS EurekAlert! news release.
Identification of a new fear circuit from the central amygdala to the globus pallidus
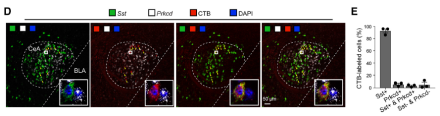
While it has long been known that the amygdala is central to fear and emotional learning, the exact organization and function of its long-range outputs remain elusive. The central amygdala (CeA) is classically thought of as the output zone of the amygdala complex, but how do CeA projections modulate fear learning? Recently, Dr. Bo Li and his team from Cold Spring Harbor Laboratory (CSHL) and Stanford University sought to answer this question. In their new paper, the researchers showed that the CeA projects to the globus pallidus (GPe) in order to relay information about a stimulus to regulate fear learning. Specifically, they found that a subpopulation of CeA-GPe projections conveys information about the unconditioned stimulus (US) during auditory fear conditioning. During fear conditioning, the US is the electrical foot shock, whereas the stimulus that elicits a fear response (i.e., freezing behavior) after it is paired with the US (i.e., a tone) is called the conditioned stimulus. Researchers were interested in the inhibitory somatostatin positive (Sst+) neurons within the CeA and whether their circuitry helps regulate fear learning. To investigate this, Dr. Li’s team used a combination of state-of-the-art neuroscience techniques such as anatomical tract tracing, optogenetic modulation, fiber photometry, in vitro electrophysiology, and fluorescent in situ hybridization in male and female mice. First, using retrograde cholera toxin B tracers, they confirmed that the CeA sends a subpopulation of neuronal projections to the GPe. Using sophisticated labeling procedures, they next showed that the vast majority of GPe-projecting CeA neurons express Sst. Anterograde tracing also demonstrated that projections from the CeA to the GPe originate predominantly from Sst+ neurons. Using a tetanus toxin light chain (TeLC) viral method, they also showed that blocking neurotransmission within CeA-GPe projections abolished conditioned freezing, indicating that these projections are necessary for fear learning. Fiber photometry revealed that these projections are specifically relaying information about the US during auditory fear conditioning. Further, optogenetically inhibiting or activating GPe-projecting CeA neurons during US presentation blocked or promoted fear learning, respectively, demonstrating that these projections are essential for fear memory formation. Altogether, this team showed that a new CeA-GPe projection is critical for fear memory regulation. These data are critical for characterizing the circuits controlling fear learning-related behaviors, which is an important step toward the development of circuit-based therapeutics following aversive experiences. Read more about this study in the CSHL news release.
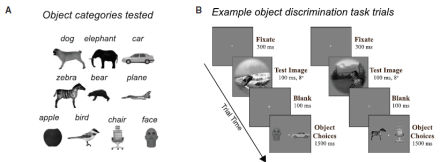
The macaque ventrolateral prefrontal cortex boosts inferior temporal cortex population coding to allow rapid object recognition
While the prefrontal cortex has long been known for its role in cognition and emotional processing, it is also critical for visual perception. There is also an emerging role for the inferior temporal cortex (IT) in object discrimination. But how do these two regions interact to enable the recognition of objects? Studying the computational functions within cortical circuits is critical in developing the next generation of models of visual intelligence, which will enable us to understand fundamental behaviors such as object recognition and discrimination. At the Massachusetts Institute of Technology, Dr. James DiCarlo and Dr. Kohitij Kar are investigating these topics. Their new study illustrates the role of connections between the ventrolateral prefrontal cortex (vlPFC) and the primate ventral visual cortex in robust core visual object recognition. Specifically, they were interested in testing whether the vlPFC is an important node in the visual object processing network by pharmacologically inactivating this region and simultaneously recording IT activity with Utah electrode arrays in macaques. They found that reversible, pharmacological inactivation of the vlPFC with muscimol reduced the quality of the IT population code and resulted in deteriorations in object discrimination performance. During the visual discrimination task, this effect of silencing the vlPFC was significantly higher for the late-solved images than for the early-solved images. They also found that inactivation of the vlPFC reduced IT late-phase neuronal population activity. These results suggest that vlPFC is part of a recurrent neural circuit that boosts the performance of the ventral visual processing stream, as opposed to shallow feedforward systems. Studies such as this will one day help to elucidate a complete, mechanistic understanding of visual object recognition, from images to behavior, at the level of the neuron. Read more about these findings in the MIT News press release.