
Researchers have genetically modified the entire family of GABAA receptor subtypes, which mediate inhibitory synaptic transmission in the brain, to make them controllable with pulses of light with high spatial, temporal, and biochemical precision.
GABA (gamma aminobutyric acid) is the main inhibitory neurotransmitter in the brain, providing a counterpoint to glutamate, the main excitatory neurotransmitter. Disruptions to the delicate balance between GABA-based inhibition and glutamate-based excitation can impede normal sensory processing, motor pattern generation, and cognitive function and result in movement disorders, epilepsy, schizophrenia, and neurodevelopmental disorders. A variety of receptor types for GABA exist, each with a unique function. Researchers have genetically engineered a portion of the receptor types that can be selectively turned on or off with brief flashes of light, called light-regulated GABA Receptors (LiGABARs). This suite of LiGABARs promises to expand the knowledge of GABA receptor function in both healthy and diseased brains, and provide a toolkit for the precise control of inhibitory synaptic transmission in the brain.
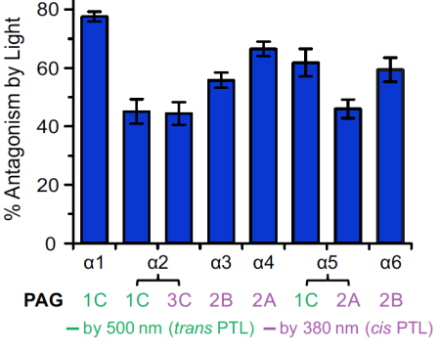
GABA exerts some of its inhibitory effects by binding to and opening chloride ion channels known as GABAA receptors. These receptors are comprised of five subunits: two alpha subunits, two beta subunits, and one tertiary subunit. The alpha subunit contributes to binding of the GABA molecule, determining when and for how long the channel opens following binding. There are six alpha subunit isoforms—each with a slightly different chemical composition, timing of expression during development, and localization within the brain.
By replacing the alpha subunits in GABAA receptors of mice with genetically engineered subunits that can be activated by pulses of light, BRAIN Initiative grantee Richard Kramer, PhD and colleagues at the University of California, Berkeley, generated light-sensitive versions of the entire GABAA receptor family. The research team reported the new light-regulated GABAA receptors in the journal Neuron.
Experiments to characterize the receptors’ performance parameters in LiGABAR knockin mice demonstrated that inhibition can be photo-controlled quickly—with a timescale of 100 to 200 ms—and with a spatial scale as deep as 350 mm from the brain surface, extending through layer 2/3 of mouse cerebral cortex.
Interestingly, some of the isoforms could be activated by light at a wavelength of 390 nm and other isoforms that were activated by light at 500 nm (Figure 1). Applying the different wavelengths of light at different times provided the opportunity to systematically characterize the distinct functions of individual GABAA receptor isoforms within a single neuron, which had not previously been possible.
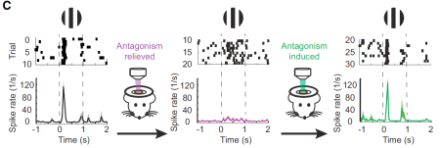
The researchers also demonstrated that LiGABARs could control in vivo visual responses in the cerebral cortex of mice (Figure 2).
In addition, the researchers argued that the LiGABAR toolkit allows for future investigations of GABAA receptor function across many spatial scales.
At the cellular level, LiGABARs can be used to determine the functions of different GABAA isoforms within a neuron. Independent photo-control offers a way to compare the regional distribution and impact of different isoforms. For example, experiments suggested that the alpah1 isoform was concentrated at synapses whereas the alpha5 isoform was more broadly distributed, consistent with prior immunolabeling studies.
At the network level, LiGABAR can help reveal the functional impact of inhibition in a neural circuit. Also, because LiGABAR can be targeted to either presynaptic or postsynaptic GABAA receptors, the relative contributions of each receptor type can be assessed under various conditions.
At the organism level, the new knockin mouse offers the unique opportunity to reversibly and specifically inhibit an endogenous neurotransmitter receptor in vivo, revealing its role in neural information processing and behavior. In addition, the GABAA knockin mice may be useful for target validation in drug discovery.
In summary the new LiGABAR toolkit will give researchers the opportunity to learn more about the role of normal GABA functioning as well as abnormal GABA signaling in movement disorders, epilepsy, schizophrenia, and neurodevelopmental disorders.
