
Genetic analysis of different populations of neural stem cells indicates that one group of cells—the outer radial glia—gives rise to the majority of neurons in the upper layers of the neocortex that are associated with higher-level information processing and thinking, suggesting that these cells are important mediators of brain evolution.
During development, neural stem cells called outer subventricular zone radial glia (oRG) can each produce hundreds of neurons of diverse types that migrate from the ventricles to the upper layers of the cortex. Although oRGs, which are located within cerebrospinal fluid-producing cavities near the center of the brain called the lateral ventricles, generate the majority of cortical neurons, the molecular features giving rise to their ability to function as stem cells are unknown. Understanding the molecular properties of oRGs can provide insights into cortical development and lead to strategies for culturing these cells in vitro. However, previous attempts to identify the genes uniquely expressed by oRGs have failed due to difficulties in isolating these cells from related stem cells. Similar to oRG, ventricular radial glia (vRG) occupy a different location within the lateral ventricles and are not as proliferative.
To classify the two cell types, Arnold Kriegstein, PhD, a neuroscientist at UC San Francisco, and colleagues used a genetic technique called single-cell RNA-sequencing to identify hundreds of the genes expressed by vRGs and oRGs. They then used a statistical technique called principal component analysis to group the cells based on their gene expression patterns. The genetic analysis, published in a paper in Cell, indicates that oRGs preferentially express genes related to the production of growth factors and other cellular support structures, as well as the ability to migrate throughout the cortex. oRGs were also observed to down-regulate classical radial glial genes and to up-regulate proneural transcription factors and neuropeptide signaling genes. Together, these molecular attributes constitute a novel transcriptional state that arises during early cortical development and facilitates neuronal differentiation and proliferation.
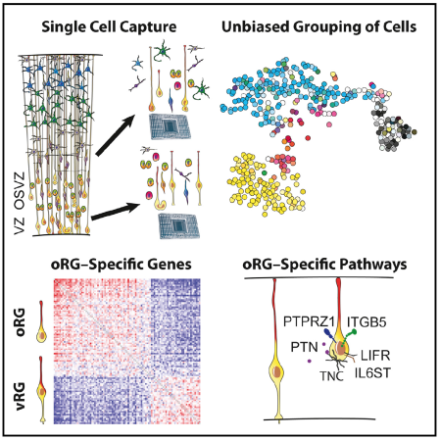
Single-cell capture techniques were used to analyze the gene expression of individual cells in the ventricular and outer subventricular zones (top left). Based on this analysis, cells were grouped into several categories including interneurons, mature neurons, intermediate progenitor neurons, newborn neurons, and vRGs and oRGs (top right). Principal component analysis was used to classify oRGs and vRGs (bottom left). Further analysis identified oRG-specific pathways important to neural differentiation and migration (bottom right). DOI:10.1016/j.cell.2015.09.004.
The researchers observed similar populations of oRGs in rhesus macaque monkeys, but oRGs were very rare in mice and they produced 10 to 100 times fewer glial and neural daughter cells than human oRGs, indicating that these cells are particularly important for the development of cortical structures that support higher brain functions. In future experiments, researchers can investigate the roles oRG genes play in neurodevelopmental disorders and trace oRG gene expression through various primates to reveal the evolution of brain features that underlie higher cognitive functions. In addition, similar techniques can be used to characterize the enormous cellular diversity within the nervous system—one of the early goals of the BRAIN Initiative.
