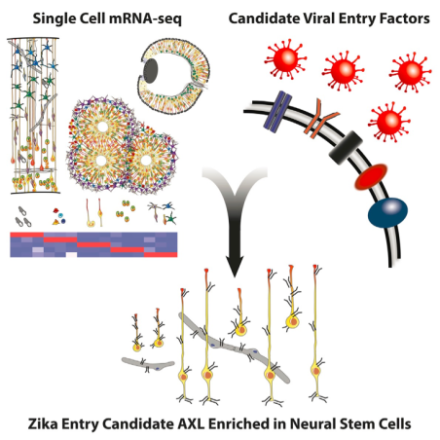
Candidate viral entry receptor for Zika Virus in neural stem cells identified… researchers create 3D topographical map of motor cortex output projections… discovery of new type of On bipolar cell and spatiotemporal wiring specificity in retinal computation of direction…
A candidate entry receptor for Zika virus in neural stem cells may be linked to microcephaly.
Zika virus was recently declared a global health emergency by the CDC because of its link to microcephaly and fetal abnormalities, though the mechanism of action is unclear. In a Brief Report in Cell Stem Cell, BRAIN Initiative grantee Dr. Arnold Kriegstein
and his colleagues at University of California, San Francisco, identified a likely connection. Primary microcephaly is thought to be caused as a result of depletion of the founder population of radial glia, though in some cases of Zika-related microcephaly, greater levels of cell death and severe ocular abnormalities have been observed, suggesting a broader effect on early brain development. Single-cell genome expression was analyzed via RNA sequencing to classify different neural cell types across early development. Kriegstein et al. surveyed expression levels of candidate Zika virus entry receptors in each cell type and discovered AXL receptor tyrosine kinase (AXL) is the most enriched candidate receptor in human radial glial cells, astrocytes, endothelial cells, and microglia in cortex. AXL expression remained high in radial glia, capillaries, and astrocytes from gestational week 13.5 to term, but was absent from more mature cells, thus identifying an important vulnerability to Zika infection. Interestingly, AXL expression was also high in neural progenitor cells of the retina, which could explain the severe ocular abnormalities observed in some cases of fetal Zika infection. Furthermore, the team observed a conservation of high AXL expression patterns in mice, ferrets, and human induced pluripotent stem cells, all of which could serve as important models to study mechanisms of Zika virus infectivity and effects on brain development.
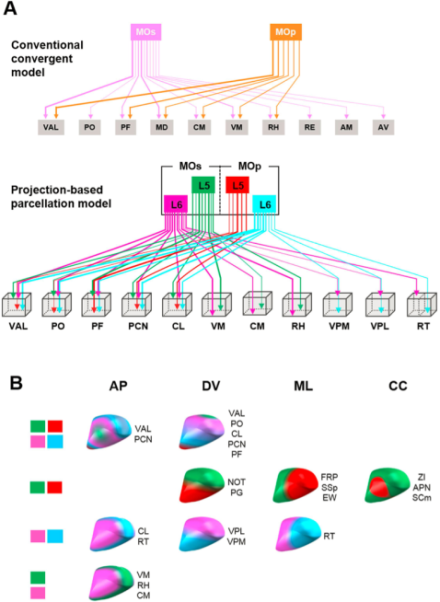
Researchers reveal a more sophisticated, systematically organized connectome of motor output projections than previously realized.
The brain’s motor cortex orchestrates a range of simple to complex motor behaviors by targeting various brain regions via output projections from primary (MOp) and secondary (MOs) motor cortices. Each of these output bundles is further divided into layer 5 and layer 6 projections, creating four distinct motor output projections whose precise network integration structure is not well known. Using a combination of whole brain serial 2-photon tomography and two genetically modified mouse lines to visualize each type of projection, BRAIN Initiative grantee Dr. Pavel Osten and his Cold Spring Harbor colleagues, in collaboration with Dr. Daesoo Kim at the Korea Advanced Institute of Science and Technology, created a 3D map showing the tract pathways and targeting locations of all four projections, described in a recent issue of Scientific Reports. Their high resolution map revealed that for common targets such as the basal ganglia, midbrain, and medulla, there are different spatial organization patterns within the same target nuclei. These subtle differences suggest the MOp and MOs projections have a sophisticated regulation over generating different combinations of muscle movements through direct and indirect activation of motor neurons. Additionally, sensory functions of the thalamus are well-documented, but motor functions are not. Generating this detailed 3D topographical map of mouse motor cortical projections enables refined identification of thalamic nuclei boundaries. This can aid understanding of motor circuit function in the mouse brain, and potentially provide insight on motor circuit dysfunction in human movement disorders.
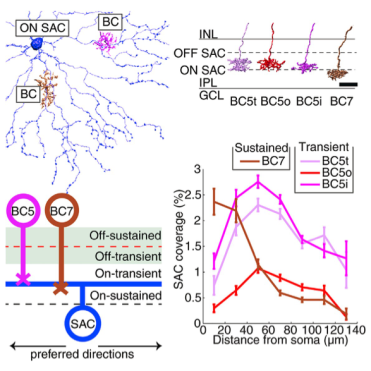
On starburst amacrine cell–bipolar cell (SAC-BC) pathway circuits confirmed to parallel spatiotemporal wiring of Off SAC-BC pathway circuits in retinal computation of moving stimuli.
Direction of motion for a visual stimulus is computed by starburst amacrine cells (SACs) and bipolar cells (BCs) in the retina, in parallel On and Off pathways. Each starburst amacrine cell’s dendritic branches are preferentially activated by visual stimuli that move outward from the soma to the dendritic tips, allowing for local computation of direction. These cells receive input from two types of bipolar cells: sustained and transient. The Off SAC-BC wiring pathways are fairly well understood, and because the Off starburst amacrine and bipolar cell types share a symmetry with the On starburst amacrine and bipolar cell types, one possibility was that the On SAC-BC pathway shares an analogous wiring mechanism with the Off SAC-BC pathway. BRAIN Initiative grantee Dr. H. Sebastian Seung , along with colleagues at MIT and Princeton University, studied the On BC-SAC circuit by reconstructing and mapping connections between On starburst amacrine and bipolar cells in the retina. This was accomplished using electron microscopy and his program EyeWire, a Citizen Science game using combined efforts of the public, to map actual neural circuits from microscopy images. In a recent publication in Cell Reports, Seung et al. reported that the On SAC-BC circuit does, in fact, parallel the Off circuit: sustained bipolar cell types prefer contact with starburst amacrine cell dendrites near the starburst amacrine cell body, and transient bipolar cell types prefer contact with dendrites farther away. This wiring mechanism could function as a correlation-type motion detector, improving our understanding of how the retina can detect and interpret motion. Additionally, during categorization of bipolar cell types, the group discovered a new type of On bipolar cell, likely completing the catalog of mouse bipolar cell types. Seung expects contact and connectivity between cells to replace stratification as the main property used to classify cells into types.